
Make the Iodine Clock Reaction (Chemistry) YouTube
The reaction is carried out in the presence of thiosulfate ion ( S 2O2 − 3) to measure the time necessary for a fixed quantity of I − 3 to be formed. The thiosulfate ion reacts rapidly with tri-iodide ion according to Reaction 4.2. I − 3 ( aq) + 2S 2O2 − 3 ( aq) 3I − ( aq) + S 4O2 − 6 ( aq)

IODINE CLOCK REACTION Instant Color Change Experiment YouTube
Iodine Clock Reaction Lab Learning Objectives Practice laboratory techniques of safely altering the temperature of a solution and creating different concentrations of a solution. Observe and record the effect of changing the temperature of a system on the rate of a reaction

The iodine clock experiment. Oscillating reaction iodineclock
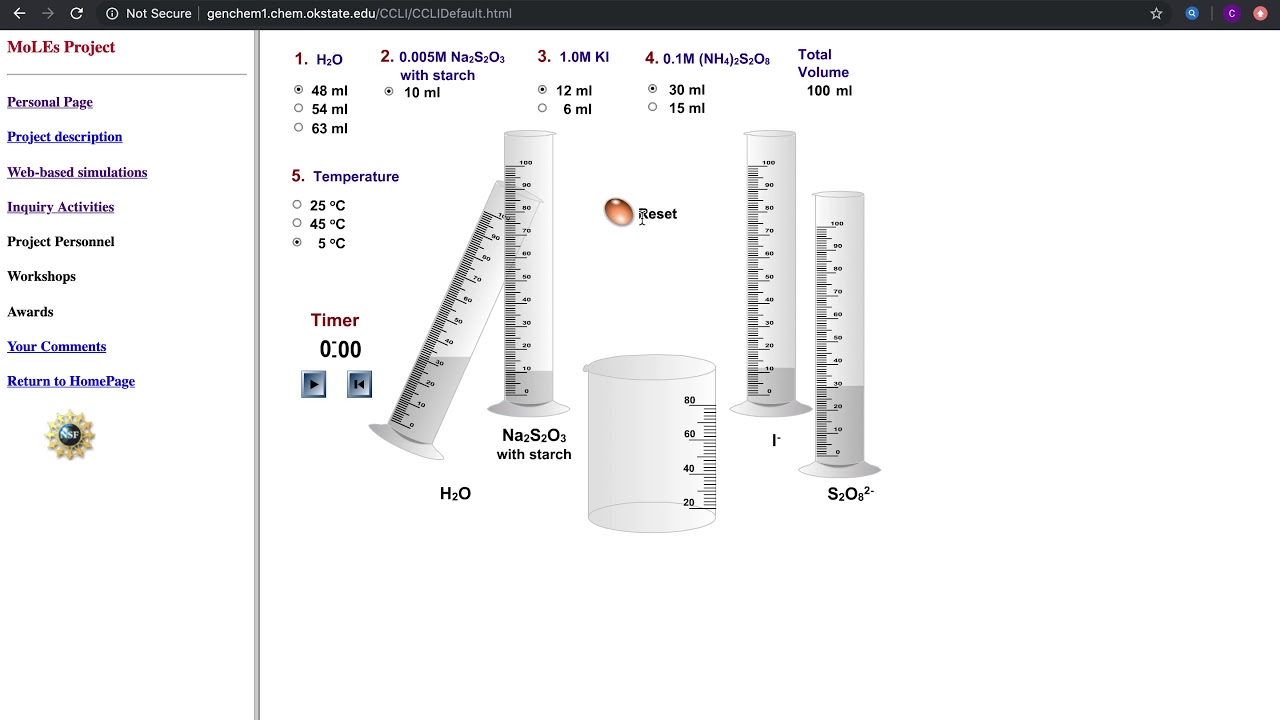
Solution Preparation Solution A 0.02 M KIO 3 Solution B 4g soluble starch, 0.2g sodium metabisulfite ( Na₂S₂O₅), 5mL 1M sulfuric acid in 1L solution Materials Electronic stop clock Ice bath in large refrigerator dish 100 mL cylinders 400 mL beakers Magnetic stirrer and stirring bars for both beakers Procedure
TemperatureDependent Iodine Clock Lecture Demonstration YouTube
The iodine clock reaction is a favorite demonstration reaction in chemistry classes that usually requires toxic or hazardous chemicals. During the reaction, two clear liquids are mixed, resulting in another clear liquid. After some time, the solution suddenly turns dark blue.

"Iodine clock" experiment (the BriggsRauscher reaction) MEL Chemistry
This iodine is immediately consumed by the thiosulfate ions (S 2 O 3 2-) in a pathway described by reaction (6). As soon as all of the S2O3 2- ions are consumed, the excess iodine produced in (5) is free to react with starch, turning the solution blue (7). The amount of thiosulfate ions added tells us how much iodine had been produced in the.

The Iodine Clock Reaction Lab ⋆ Chemistry
Experiment 15 . Iodine Clock . E15-2 . The Task . The goal of this experiment is to identify the rate-determining step in a complex reaction mechanism. Skills At the end of the laboratory session you should be able to: • determine the concentration dependence of the rate of a reaction via the method of

Iodine Clock Reaction (8.1.5) AQA A Level Chemistry Revision Notes
The purpose of this experiment was to find the Rate Law Equation, the Rate Law Constant, and the rate orders of the reactants in the reaction between two solutions; Solution A and Solution B. Solution A consisted of hydrogen peroxide, hydrogen ions, and water, and Solution B consisted of iodine ions, thiosulfate ions, and starch.

Recreating the Iodine Clock Reaction at Home with Vitamin C YouTube
A Sample Lab Report The Iodine Clock Reaction Introduction: The factors that affect the rate of a chemical reaction are important to understand due to the importance of many such reactions to our health, well-being and comfort.

iodine clock lab YouTube
Procedure: In a 400 mL beaker, add 100 mL 0.1M KIO 3, 5 mL 1% starch, and 100 mL H 2 O. In a 600 mL beaker, put in 20 mL 0.25M NaHSO 3, and 130 mL H 2 O. Mix the two solutions and after a short delay, the clear solution will instantly turn a dark blue/black ( ~ 10 seconds)

of the Iodine Clock Reaction Intro & Theory YouTube
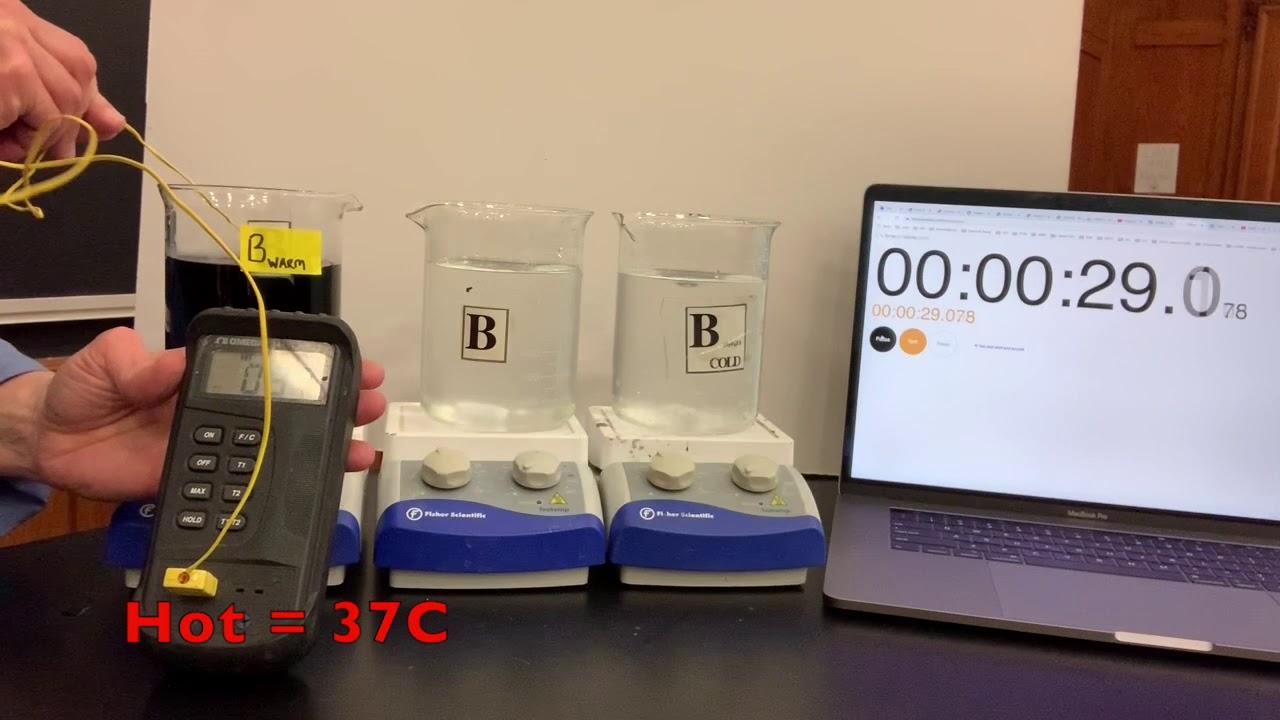
Obtain and mix the reagents in the same volumes as in step 1 for the room temperature solution. Step 2. Place the beaker in a water bath with ice. Measure the temperature on the solution. Leave the solution in the bath until the temperature is at least 10o 10 o C colder than the room temperature solution. Step 3.

Lab Demo Iodine Clock Reaction (Chemical Change) YouTube
Elizabeth Teper CHEM 106 12/ Lab 19: The Iodine Clock-A Chemical Kinetics Lab. Introduction: The iodine clock reaction is an experiment that found out that a reaction does not always have to react right away but can occur after a certain amount of time. In this lab, we will be determining the rate constant and the activation energy of persulfate.
iodine clock lab YouTube
Introduce your students to rates of reaction and kinetics with the iodine 'clock' reaction. Mix a solution of hydrogen peroxide with potassium iodide, starch and sodium thiosulfate to see a colourless solution suddenly turn dark blue This demonstration can be used at secondary level as an introduction to some of the ideas about kinetics.

The Iodine Clock Reaction Lab ⋆ Chemistry Lab
The iodine clock reaction exists in several variations, which each involve iodine species ( iodide ion, free iodine, or iodate ion) and redox reagents in the presence of starch. Two colourless solutions are mixed and at first there is no visible reaction.

The Iodine Clock Reaction Lab ⋆ Chemistry
Determine the rate law and activation energy of an iodine clock reaction. Learning Outcomes Calculate the rate of reaction given experimental data. Use the method of initial rates to determine the rate law and rate constant for a reaction. Graphically determine the activation energy of a reaction using the Arrhenius equation. Introduction

Classic Chemistry Colorize Colorless Liquids with "Black" Magic, AKA
17 Kinetics of the Iodine Clock Reaction Purpose To determine the rate law and activation energy of a iodine clock reaction. Expected Learning Outcomes After completing this experiment, it is expected that students will be able to Determine the rate law and rate constant of a reaction.

How to Perform the Iodine Clock Reaction 11 Steps (with Pictures)
Solution A: 2.1 g potassium iodate (V) is dissolved in 1 dm 3 deionised water followed by the addition of 10 cm 3 of 1 mol dm -3 sulfuric acid. Solution B: 4 g of soluble starch is made up into a paste with a little cold water and this is added to 1 dm 3 boiling deionised water. 0.9 g of sodium hydrogensulfite and 10 cm 3 of 1 mol dm -3.